HB XB Interactions
Interaction Types
A molecule consists of a set of atoms united by covalent bonds. When molecules come into contact, forces allow them to attract and regroup to a certain extent. These forces, called intermolecular forces, are however weaker than those involved in a covalent bond and are therefore more easily broken (Table 1). (ref.1) On the other hand, they are important enough to contribute significantly to physicochemical properties of the material.
| kJ/mol | Kcal/mol | Directionality | ||
|---|---|---|---|---|
| Intramolecular Forces (force that binds together the atoms making up a molecule) | Covalent bond | 150-1100 | 35-260 | yes |
| Intermolecular Forces (forces which mediate interaction between molecules) | Hydrogen bond (HB) | 4-50 | 1-12 (0.5 to 40) | yes |
 |
||||
| Halogen bond (XB) | 4-50 | 1-12 (0.5 to 40) | yes | |
| van der Waals interaction
(Debye, London, etc.) |
<1-25 | <1-6 | no | |
| Intramolecular Forces (force that binds together the atoms making up a molecule) | |
| Covalent bond | |
| kJ/mol | Kcal/mol |
|---|---|
| 150-1100 | 35-260 |
| Directionality | |
| yes | |
| Intermolecular Forces (forces which mediate interaction between molecules) | |
| Hydrogen bond (HB) | |
| kJ/mol | Kcal/mol |
|---|---|
| 4-50 | 1-12 (0.5 to 40) |
| Directionality | |
| yes | |
 |
| Halogen bond (XB) | |
| kJ/mol | Kcal/mol |
|---|---|
| 4-50 | 1-12 (0.5 to 40) |
| Directionality | |
| yes | |
|
van der Waals interaction
(Debye, London, etc.) |
|
| kJ/mol | Kcal/mol |
|---|---|
| <1-25 | <1-6 |
| Directionality | |
| no | |
Table 1. Comparing the strength of covalent bonds, intermolecular hydrogen and halogen bonds and vdW interactions for organic molecules (approximate values).
A particularity of the two largest intermolecular forces (hydrogen & halogen bond) is that they have some features of covalent bonding: they are directional, stronger than a van der Waals force interaction and produces interatomic distances shorter than the sum of van der Waals radius
Therefore, the molecules of matter that undergo this type of interaction tend to align with each other to favor, as far as possible, their contact geometry (angle, distance). These directional forces can sometimes create materials with unique properties, such as Kevlar, in which molecules align themselves side by side into fibers that are stabilized by inter-chain hydrogen bonds, which gives Kevlar great rigidity.
Therefore, the molecules of matter that undergo this type of interaction tend to align with each other to favor, as far as possible, their contact geometry (angle, distance). These directional forces can sometimes create materials with unique properties, such as Kevlar, in which molecules align themselves side by side into fibers that are stabilized by inter-chain hydrogen bonds, which gives Kevlar great rigidity.
Hydrogen bond Definition
The classic definition of the Hydrogen Bond (HB), which is still widely used in college and university volumes, comes from the IUPAC (International Union of Pure and Applied Chemistry) Compendium of Chemical Terminology 2nd Edition (in 1997).
Curent definition of Hydrogen Bond according to IUPAC Compendium of Chemical Terminology 2nd Edition (1997)
‘’A form of association between an electronegative atom and a hydrogen atom attached to a second, relatively electronegative atom.[…]Both electronegative atoms are usually (but not necessarily) from the first row of the Periodic Table, i.e. N, O or F. Hydrogen bonds may be intermolecular or intramolecular. With a few exceptions, usually involving fluorine, the associated energies are less than 20 - 25 kJ mol −1 (5 - 6 kcal mol −1).’’ (ref.1)
On the other hand, several observations made by chemists confirm that the definition needs to be revised to incorporate a wider range of hydrogen bonds. It should be known that since 2005, recommendations have been made to IUPAC to describe ''unconventional hydrogen bonds'' often weaker (or sometimes conversely stronger) than the conventional ones.
‘’A form of association between an electronegative atom and a hydrogen atom attached to a second, relatively electronegative atom.[…]Both electronegative atoms are usually (but not necessarily) from the first row of the Periodic Table, i.e. N, O or F. Hydrogen bonds may be intermolecular or intramolecular. With a few exceptions, usually involving fluorine, the associated energies are less than 20 - 25 kJ mol −1 (5 - 6 kcal mol −1).’’ (ref.1)
On the other hand, several observations made by chemists confirm that the definition needs to be revised to incorporate a wider range of hydrogen bonds. It should be known that since 2005, recommendations have been made to IUPAC to describe ''unconventional hydrogen bonds'' often weaker (or sometimes conversely stronger) than the conventional ones.
The proposed new definition of Hydrogen Bond (IUPAC Recommendations 2011)
‘’The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment X–H in which X is more electronegative than H, and an atom or a group of atoms in the same or a different molecule, in which there is evidence of bond formation.‘’ (ref.1) , (ref.2)
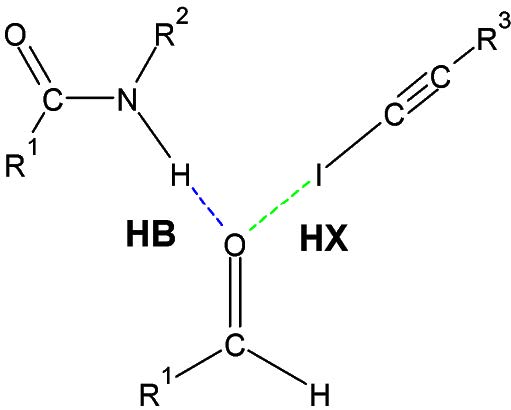
In this new definition, the atom X is not restricted to the atoms O, N or F, because, for example, an atom of C is also a more electronegative atom than an H. It is thus possible to have Hydrogen Bond like C-H...O (weaker). The evidence of the presence of the HB can be described for example by the formation of a contact which is smaller than the sum of the vDW interactions of the atoms involved and also by the directional character (example: an angle that tends to 180o) . By studying the crystallographic databases, we can see these trends (ref.1). This new definition of the HB broadens the interaction domain of the latter at energies ranging from 0.5 to 40 kcal/mol. (ref.2), (ref.3)
‘’The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment X–H in which X is more electronegative than H, and an atom or a group of atoms in the same or a different molecule, in which there is evidence of bond formation.‘’ (ref.1) , (ref.2)
In this new definition, the atom X is not restricted to the atoms O, N or F, because, for example, an atom of C is also a more electronegative atom than an H. It is thus possible to have Hydrogen Bond like C-H...O (weaker). The evidence of the presence of the HB can be described for example by the formation of a contact which is smaller than the sum of the vDW interactions of the atoms involved and also by the directional character (example: an angle that tends to 180o) . By studying the crystallographic databases, we can see these trends (ref.1). This new definition of the HB broadens the interaction domain of the latter at energies ranging from 0.5 to 40 kcal/mol. (ref.2), (ref.3)
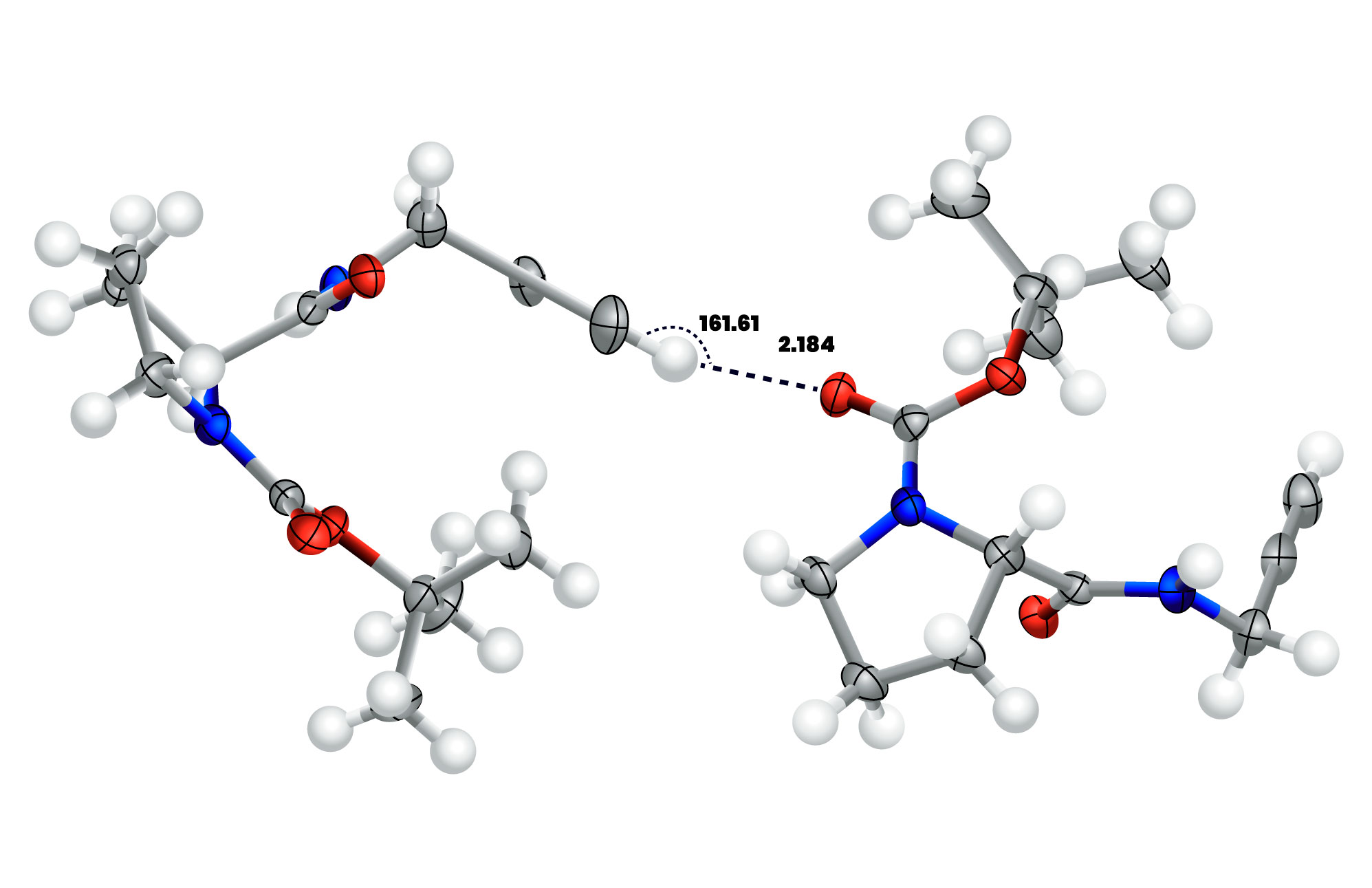
Figure 1. Geometrical parameters of the CC−H⋯O=C hydrogen bond in a proline derivative. (ref.1) The CH⋯O angle) is near linearity with a value of 161.61° and the H⋯O distance (2.184Å) is less than the sum of the vDW radii of H and O (1.20Å (for H) + 1.52Å (for O) ≅ 2.72Å).
Halogen bond DEFINITION
Strangely similar to the hydrogen bond, the halogen bond is yet unknown and absent from the volumes of chemistry college and university. We are the first authors to introduce the halogen bond in a volume of organic chemistry in Quebec. The reason is that it is counterintuitive to think that a halogen atom (a relatively electronegative atom) can have an electrophilic region and thus act as a hydrogen bond.
The presence of the halogen interaction in the scientific literature was thus more discreet than in the case of the Hydrogen bond. The work of Politzer and Murray in the 1990s made a significant contribution in describing the electrophilic region involved in a halogen bond, a region known as the sigma hole (figure 2). (ref.2)
The presence of the halogen interaction in the scientific literature was thus more discreet than in the case of the Hydrogen bond. The work of Politzer and Murray in the 1990s made a significant contribution in describing the electrophilic region involved in a halogen bond, a region known as the sigma hole (figure 2). (ref.2)
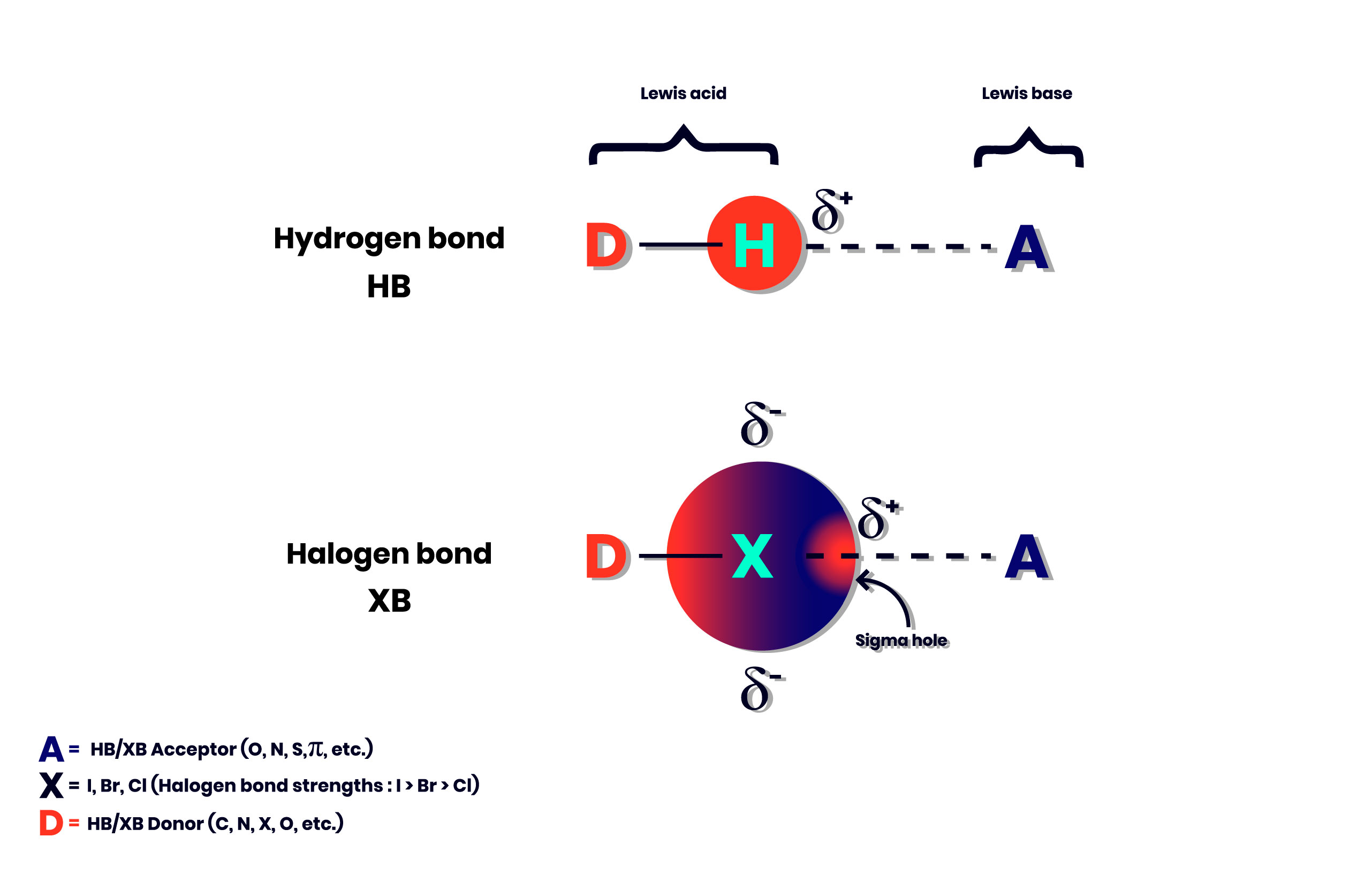
Figure 2. Differences and similarities between Hydrogen and Halogen bond.
Like the Hydrogen Bond, the energies involved can be very variable ranging from 10 kJ/mol (example a contact N···Cl)(ref.1) to 150 kJ/mol (example a contact I2···I–). (ref.2)
There is currently no official definition of IUPAC, but instead a 2013 recommendation that originated in a project that began in 2009. This project was initiated by the IUPAC group, having the aim “to take a comprehensive look at intermolecular interactions involving halogens as electrophilic species and classify them”. (ref.3)
There is currently no official definition of IUPAC, but instead a 2013 recommendation that originated in a project that began in 2009. This project was initiated by the IUPAC group, having the aim “to take a comprehensive look at intermolecular interactions involving halogens as electrophilic species and classify them”. (ref.3)
Definition of the halogen bond (IUPAC Recommendations 2013) (ref.1)
‘’A halogen bond occurs when there is evidence of a net attractive interaction between an electrophilic region associated with a halogen atom in a molecular entity and a nucleophilic region in another, or the same, molecular entity.‘’ (ref.2)
‘’A halogen bond occurs when there is evidence of a net attractive interaction between an electrophilic region associated with a halogen atom in a molecular entity and a nucleophilic region in another, or the same, molecular entity.‘’ (ref.2)
Despite the similarities with the Hydrogen Bond, four differences can be highlighted between the halogen bond and the latter. (ref.1)
- Halogen bonds tend to be more directional than Hydrogen bond.
- In the case of halogen bonds, the strength of the interaction can be tuned. In order, the most polarizable halogen atoms (I> Br> Cl) form the strongest bonds.
- The halogen bonds are hydrophobic unlike the Hydrogen bond which are hydrophilic.
- The size of the halogen atom is considerably larger than an atom of H, which offers chemists more possibilities for the design of functional materials. On the other hand, the steric hindrance is sometimes a problem.